FDA Issues Warning Against Reumofan Plus
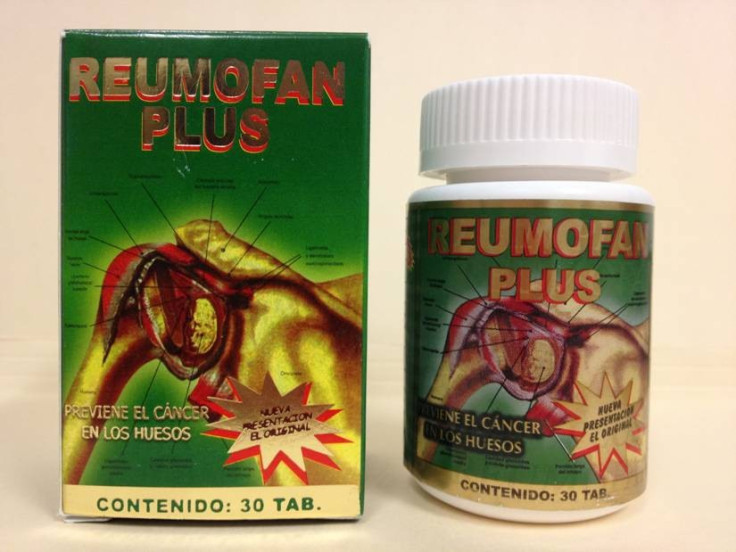
The U.S. Food and Drug Administration (FDA) has issued a warning for consumers against the purchase of Reumofan Plus which is sold as a "natural" dietary supplement for pain relief and other serious conditions.
The drug reportedly contains several active pharmaceutical ingredients not listed on the label that could be harmful. Labeled in Spanish, the drug is promoted for the treatment of several conditions such as arthritis, muscle pain, osteoporosis and bone cancer.
The product is manufactured in Mexico by Riger Naturals and sold in some retail outlets, flea markets, and on various internet sites. FDA has worked closely with the Mexican government on this matter. The Mexican Ministry of Health has issued a health warning to the public and ordered Riger Naturals to recall the product.
A number of adverse effects have been cited by FDA associated with the use of the drug which includes liver injury, sudden worsening of glucose (sugar) control, weight gain, swelling, leg cramps, and adrenal suppression.
An FDA laboratory analysis of Reumofan Plus found that it contains methocarbamol which is a prescription muscle relaxant that can cause sedation, dizziness, low blood pressure, and impair mental or physical abilities to perform tasks such as driving a motor vehicle or operating machinery.
Additionally, diclofenac sodium which is a prescription non-steroidal anti-inflammatory drug (NSAID) was also found which may cause increased risk of cardiovascular events such as heart attack and stroke, as well as serious gastrointestinal (GI) adverse events.
These ingredients also may interact with other medications and result in serious adverse events. The Mexican Ministry of Health discovered that at least one lot of the product contains the corticosteroid dexamethasone, a drug that acts as an anti-inflammatory and immune system suppressant.
According to FDA adverse events reports, consumers reported symptoms suggesting that some lots of Reumofan Plus may contain corticosteroids. Abrupt discontinuation of corticosteroids after long-term or high dose use can cause fatigue, nausea, low blood pressure, low blood sugar levels, fever, muscle, and joint pain, dizziness, and fainting.
© Copyright IBTimes 2025. All rights reserved.