'Invisible' hydrogel films could regenerate cornea cells and improve transplantation outcomes
The new technique could help millions of people worldwide who suffer from vision problems.
Australian researchers have successfully grown and implanted cornea cells in animal models, paving the way for clinical trials in humans. Their technique, which consists in growing cells on a specially developed synthetic hydrogel film that could then be implanted into a patient's eye, could help the 10 million people worldwide who are affected by corneal disease.
The Melbourne University-led team of scientists focused on a specific set of cells in the cornea called "corneal endothelial cells" – a single layer of cells on the inner surface of the cornea. Their function is to keep the cornea clear, pumping out excess fluid out of the cornea so that it does not swell with water and become thick and opaque.
The problem is that trauma, disease, or more commonly, ageing, can reduce the number of these endothelial cells overtime. This can lead to vision deterioration, leaving doctors with only one therapeutic option: implanting their patients with donors' cornea.
Real added-value
This is problematic for a number of reason. First, there is a global shortage of donor's cornea. Furthermore, the procedure is risky, as it can promote disease transmission or lead to the rejection of the donor's cornea by the immune system. In China, doctors have been experimenting with pig corneas, but with animal tissue, the risk of it being rejected as a foreign body by the patients is even greater.
With the new hydrogel film technique, this is exactly the issues that the scientists seek to avoid. "The issue with donor tissue is that the whole process from handling, harvesting cells from the patient's, storing and then transplanting them can have detrimental effects on the cells themselves. There is a potential risk for disease transmission and a risk of tissue rejection, since you are transplanting from a foreign body," Research scientist Berkay Ozcelik told IBTimes UK.
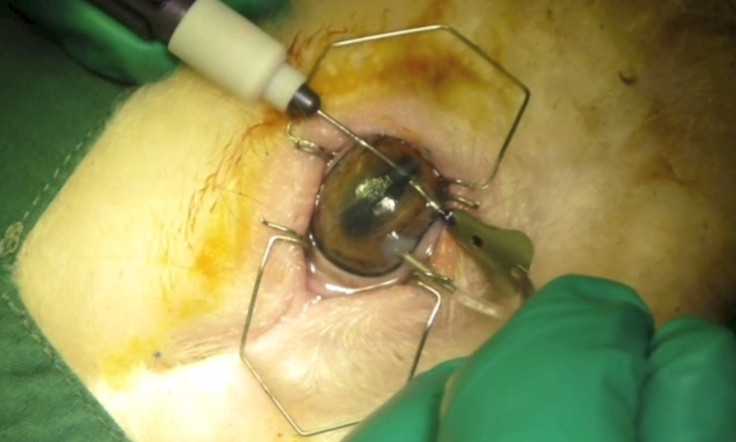
"Our ultimate aim is to use patients' own cells to regenerate them on the hydrogel films we have developed, and to implant them directly in the patient's cornea. Since it's their own cells we are using, there is no risk of disease transmission or tissue rejection."
And even if the scientists do not end up using the patient's own cells, the technique has a real added-value, as it could enable more people to be cured from the same donor.
"The other advantage of our technique even if you don't use patient's own cells, because we can regenerate and increase the number of a donor's cells in culture, we could use cornea material from one donor for maybe, say, 20 patients", Ozcelik adds.
Future clinical trials
Working with the Centre for Eye Research Australia, the scientists have so far carried out their experimentation in sheep – a large animal model with a cornea sharing many similarities with humans. The cells are removed from the animal's cornea and and grown on very thin hydrogel films (50-μm thin). Once the cells have been regenerated in this way, the surgical procedure to implant the almost "invisible" film only requires a small incision in the cornea and a simple insertion through that incision.
The hydrogel film is naturally able to adhere into the cornea and allow the cells to function – restoring the cornea's vital water-pumping activity, so that it becomes once more becomes transparent.
This approach has been successful in sheep with the findings published in the journal Advanced Healthcare Material. The next step is now to secure funding and approval for clinical trials in humans in coming years.
© Copyright IBTimes 2024. All rights reserved.







