MIT scientists find way to make lithium-ion batteries last indefinitely and store more energy
MIT scientists have succeeded in making not one but two significant breakthroughs that can potentially not only give lithium-ion batteries almost indefinite lifetimes, but also quadruple the amount of energy these batteries can store.
Lithium-ion batteries are used today in smartphones, tablets, cameras, laptops, electric cars, planes and boats and are already able to store a great deal of energy in a small container, but scientists continue to work to make them even better.
And add to that, there's the issue that there have been cases of lithium-ion batteries in smartphones overheating and exploding, so finding a better solution is key.
Developing solid state batteries that aren't flammable
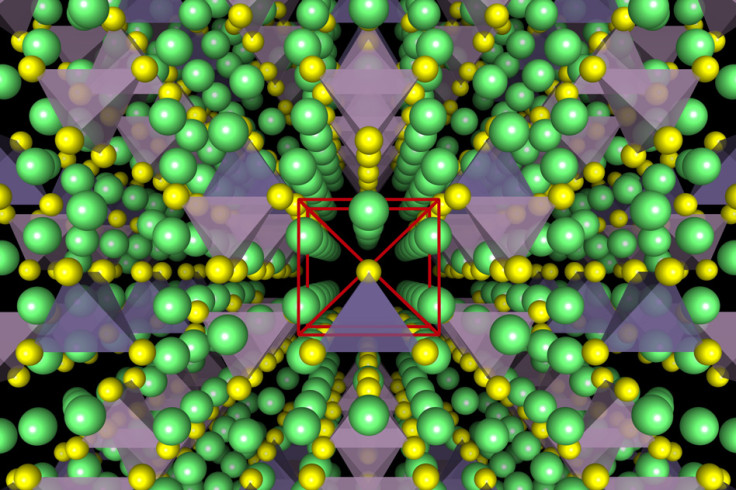
One of the three basic components in batteries is the electrolyte, and at the moment, most rechargeable batteries contain a liquid electrolyte, which is flammable and responsible for the overheating, so researchers from MIT, Samsung, University of California at San Diego and the University of Maryland decided to develop a solid electrolyte instead.
They developed a solid lithium-ion conductor made from a compound of lithium, germanium, phosphorus and sulphur that was a workable solid-state electrolyte.
Not only is the solid-state electrolyte safer as it isn't flammable, but it also lasts much longer than a conventional battery as it has no degradation reactions left. This means that the batteries would survive being used, recharged and used again for hundreds of thousands of cycles.
Even better, batteries that included the solid-state electrolyte were able to perform even at extremely cold temperatures of -20 degrees Fahrenheit, which isn't possible in the batteries of today.
The research, entitled Design principles for solid-state lithium superionic conductors is published in the journal Nature Materials.
Forgetting about an experiment led to a breakthrough
In a separate study, MIT and Tsinghua University scientists accidentally discovered that they could quadruple the amount of storage available in a lithium-ion battery after leaving an experiment running for too long.
Over time, lithium-ion rechargeable batteries begin to decline in performance as lithium compounds build up on the electrodes as they charge and discharge. This exposes the electrodes which eventually decompose.
A solution would be to replace the graphite anodes being used today with aluminium, but oxide forms immediately on the surface of aluminium nanoparticles as soon as they are exposed to air.
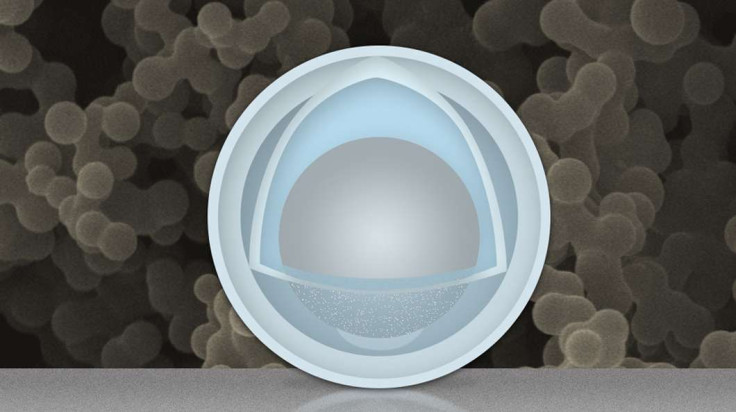
So Dr Wang Changan of Tsinghua University and Dr Li Ju of MIT decided to soak aluminium nanoparticles in a mix of sulphuric acid and titanium oxysulphate, in order to remove aluminium oxide and replace it with a new outer coating of titanium oxide.
However, they forgot about one batch of nanoparticles and left it to soak for so long that the mixture soaked into the aluminium nanoparticles. The nanoparticles were originally 50nm of aluminium, but after being soaked, the nanoparticle then had an inner "yolk" of only 30nm of aluminium, while the shell that covered it was 4nm of titanium hydroxide.
The scientists decided to use these new nanoparticles to build batteries, and they created a battery that was able to retain four times the amount of energy that current batteries are able to store, as well as lengthening the battery's lifespan by over 500 use and recharge cycles.
Their paper, entitled "High-rate aluminium yolk-shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity" is published in the journal Nature Communications.
© Copyright IBTimes 2025. All rights reserved.






















