3D printing medical breakthrough could help bones regenerate without the use of grafts
Trinity College Dublin scientists use stem cells and bio materials to construct templates of missing bones.
Irish scientists have made a medical breakthrough by developing a method to make new bones using 3D printing in order to fix serious injuries and bone defects, rather than by using painful bone grafts.
Bioengineers from the Science Foundation Ireland-funded AMBER materials science centre at Trinity College Dublin have developed a new technique of 3D bioprinting new cartilage templates in the shape of missing bones, which can then be implanted and used by the body to grow new bones.
3D printing has been around for more than two decades, known in the manufacturing industry as "additive manufacturing" or "rapid prototyping" – a way to produce objects quickly without needing to wait weeks to receive the finished product from a factory in China. It has become increasingly popular over the last two years with consumers and other industries, such as automobiles and aerospace.
3D printing in medicine is also a rising field, although typically this involves printing prosthetics and modelling patients' bodies before particularly challenging surgeries in order to make sure that tumours can be removed accurately without causing damage to nearby nerves and tissues. There is also bioprinting, but this is yet another completely different ball park.
How the 3D printed bone technique works
A combination of stem cells is printed in a hydrogel using a RegenHU 3DDiscovery multi-head bioprinter. While one extruder prints the stem cells, a stiffer, supporting bio material is printed beside the cells in the hydrogel, layer by layer, until the process is complete.
The structure is then placed in an incubator for four weeks together with molecules that tell the stem cells to turn into cartilage. Four weeks later, the 3D printed structure turns into a cartilage template and it can then be implanted under the skin.
The cartilage goes through a process called vascularisation, whereby blood vessels develop in the bone tissue, which eventually makes it possible for the missing bone to grow back naturally.
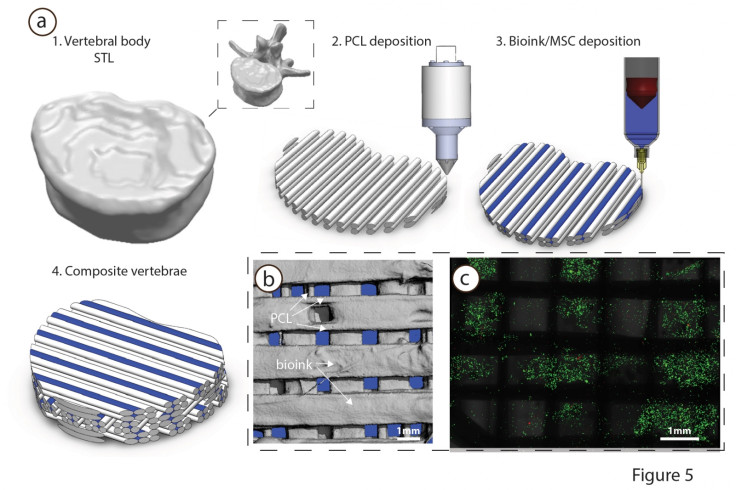
Bioprinting could greatly transform and revolutionise the future of medicine, but the technology is still in its infancy, as there are still a myriad of challenges involved in 3D printing human tissues that make it impossible even to think about printing organs.
"The problem with bioprinting at the moment is that we can't print any complex structures, tissues or organs. If we try to print bone tissues, there's a complex blood network inside it. We feel at the moment, for complex tissues, this is a different approach that, at least based on current technology, is a better approach to adopt for 3D bioprinting," Professor Daniel Kelly, director of the Trinity Centre for Bioengineering (TCBE) at Trinity College Dublin told IBTimes UK.
"Instead of trying to directly print a bone tissue, our bones start off as a condensation of stem cells. This condensation turns into cartilage, a softer tissue. This tissue is much simpler than adult bone. This cartilage tissue is a template and the bone forms on or within this template."
The challenges in regenerating bones
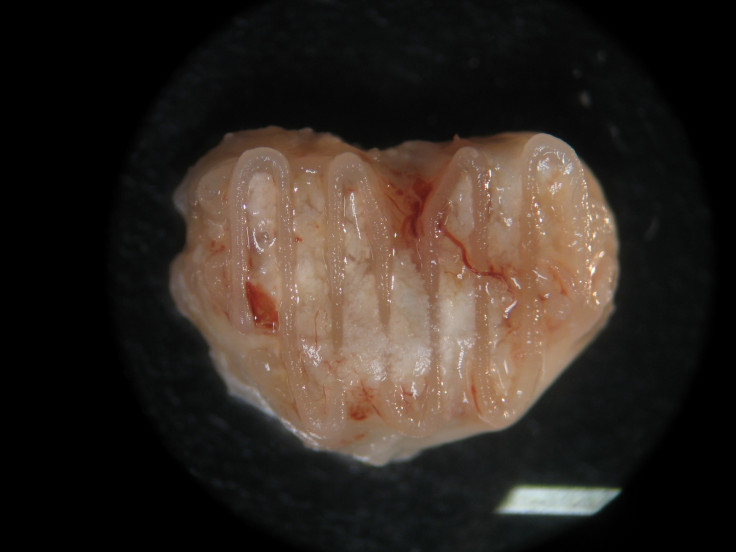
And while this still sounds pretty amazing, in reality bone regeneration is a really slow process and the scientists do not yet know how long it would feasibly take to regrow a bone in this way, as they have only succeeded in getting the bone to grow in a mouse using cells from a pig.
"It's hard to say at this stage how long it will take. Our goal would be to create a graft that has comparable rates of healing to auto-grafting, but isn't as painful, and [eventually] to think about treating diseases like osteoarthritis by printing cartilage so we can look to revive diseased joints with biological implants," said Kelly, who led the research.
"One of the reasons we've moved to 3D printing is that you can print any shape, any size. The next challenge is; can you grow a very large piece of cartilage? To do that, we need to develop a special bioreactor, which controls the environment of the cells to ensure that they all stay alive, and we are developing this in parallel."
The researchers are in the third year of their five-year project, and the technology will only likely become available in about five to 10 years' time.
The paper, entitled 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering, was presented by Professor Daniel Kelly at the Five-Year Trinity Biomedical Sciences Institute (TBSI) Symposium in Dublin on Monday (5 September).
© Copyright IBTimes 2025. All rights reserved.






















