Chemosynthetic Livers: an End to Animal Testing in Pharmaceutical Industry?

A new method of testing drugs has been developed by scientists – without the use of animals.
Presented at the 247th National Meeting & Exposition of the American Chemical Society, scientists say they have developed a method of determining drug safety and drug interactions without testing on animals.
Chemosynthetic livers are catalysts that act similarly to an enzyme known as cytochrome P450. These catalysts are substances that speed up processes that either would not happen or would occur very slowly.
Mukund Chorghade, chief scientific officer of drug research company Empiriko Corporation, said: "Researchers in drug discovery make small quantities of new potential drug compounds and then test them in animals. It is a very painstaking, laborious and costly process. Frequently, scientists have to sacrifice many animals, and even after all that, the results are not optimal."
Normally, to test a new drug, scientists test the compound on animals to find out if it is toxic before taking it to the clinical trial stage.
This is done through a process called metabolic profiling – after an animal is given the drug, the scientists look to see if it does what it is supposed to until it is broken down by the liver.
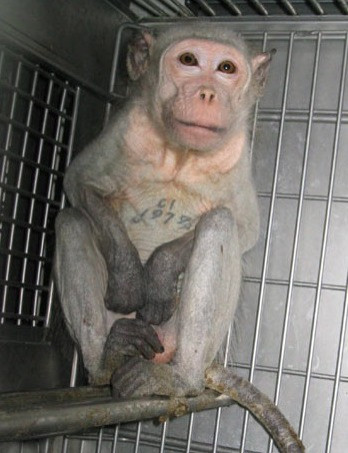
Researchers then look for molecular byproducts, which are often responsible for horrible side effects that prevent a potential drug being used on humans.
Instead of testing on animals, chemosynthetic livers act as stand-ins, so researchers can work out metabolic profiles of drugs by mixing them in test tubes.
"These chemosynthetic livers not only produce the same metabolites as live animals in a fraction of the time," Chorghade said, "but they also provide a more comprehensive metabolic profile, in far larger quantities for further testing and analysis."
Chemosynthetic livers, or Biomimiks, have not yet been approved to replace animal testing, but the team is optimistic about their future. Chorghade said so far they have tested 50 drugs and the catalysts have accurately mimicked how the human body would process them.
Once they have tested 100 drugs, they will be able to take Biomimiks to the US Food and Drug Administration requires for regulatory approval.
As well as putting an end to animal testing, Chorghade said Biomimiks could be used to see how different drugs react with one another when a person is taking multiple drugs.
Chorghade said: "The average American above 60 years of age is taking multiple drugs a day. Side effects from drug-to-drug interactions could be substantial."
In response to the announcement, a spokesman for Peta said: "Today – because experiments on animals are cruel, expensive and generally inapplicable to humans – the world's most forward-thinking scientists have moved on to develop and use methods for studying diseases and testing drugs that replace the use of animals and are actually relevant to human health.
"Studies have repeatedly shown that these new methodologies are better at modelling human diseases and predicting how drugs will act than crude animal tests, yet the government and many health charities still waste billions of pounds from taxpayers and generous donors on cruel and misleading animal experiments.
"With greater investment in exciting and progressive non-animal methods, such as chemosynthetic livers, we'd have far more promising cures and treatments and far fewer dead animals."
© Copyright IBTimes 2025. All rights reserved.






















