Gene editing: FDA approves wonder cancer treatment in a historic first
The experimental therapy showed an astonishing 83% remission rate in clinical trials.
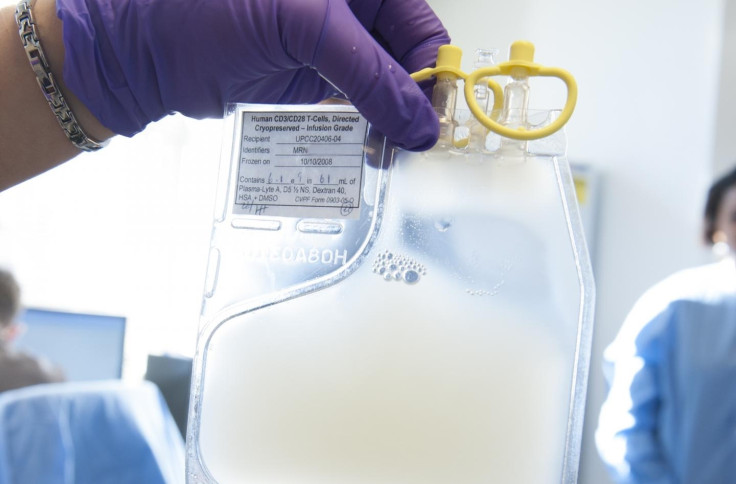
In a historic move, the US Food and Drug Administration (FDA) have approved a new gene therapy treatment for the first time. Kymriah, as it is known, is used to treat children and young adults with a rare form of leukaemia.
The therapy will now be available to patients across the US who have been diagnosed with acute lymphoblastic leukemia (ALL) and have no other treatment options available to them. Remarkably, more than 80% of patients experienced remission in clinical trials.
"We're entering a new frontier in medical innovation with the ability to reprogram a patient's own cells to attack a deadly cancer," said FDA Commissioner Scott Gottlieb. "New technologies such as gene and cell therapies hold out the potential to transform medicine and create an inflection point in our ability to treat and even cure many intractable illnesses."
Gene therapies are a group of experimental techniques which either replace or introduce genetic material into a patients' cells to treat a variety of diseases. Despite the effectiveness of many gene therapies, they are still controversial due to some of the risks involved.
Kymriah, for example, can have life threatening side effects and consequently, the FDA is requiring that all hospitals and associated clinics which will provide the treatment must be specially certified in order to mitigate the risks.
Dr Carl June, from the University of Pennsylvania, who led the research into the new drug said: "This is a turning point in the fight against ALL that opens up opportunities for patients across the world who desperately need new options."
The Kymriah treatment involves collecting patients' T-cells – crucial components of the body's immune system – and reprogramming them in a lab before reintroducing them into the body where they immediately begin 'hunting' and destroying leukemia cells.
"This approval by the FDA of Kymriah is a major milestone in the successful treatment of cancer," said John Walter, CEO of the Alliance for Cancer Gene Therapy who funded the research. "This is the first-ever true gene therapy treatment made available to the US population and will help accelerate the speed at which we will see even more gene-based therapies come to fruition. It's a very exciting time."
© Copyright IBTimes 2025. All rights reserved.





















