India approves two Covid-19 vaccines for emergency use
The two vaccines "are being approved for restricted use in emergency situations," V.G. Somani, the drugs controller general of India, said at a briefing.
India on Sunday authorised the emergency use of two coronavirus vaccines developed by AstraZeneca and Oxford University and by local pharmaceutical firm Bharat Biotech, the country's drug regulator said, paving the way for one of the world's biggest inoculation drives.
The two vaccines "are being approved for restricted use in emergency situations," V.G. Somani, the drugs controller general of India, said at a briefing.

Prime Minister Narendra Modi tweeted that the fast-track approvals were "a decisive turning point to strengthen a spirited fight" that "accelerates the road to a healthier and Covid-free nation".
India is the world's second most infected nation with more than 10.3 million cases and almost 150,000 deaths, although its rate of infection has come down significantly from a mid-September peak of more than 90,000 cases daily.

The government has already held nationwide drills ahead of the mass vaccination drive and some 96,000 health workers have been trained to administer the shots.
India has set an ambitious target of inoculating 300 million of its 1.3 billion people by mid-2021.

The World Health Organisation welcomed the news.
"The use of vaccine(s) in prioritised populations, along with continued implementation of other public health measures and community participation will be important in reducing the impact of Covid-19," WHO regional director Poonam Khetrapal Singh said in a statement.
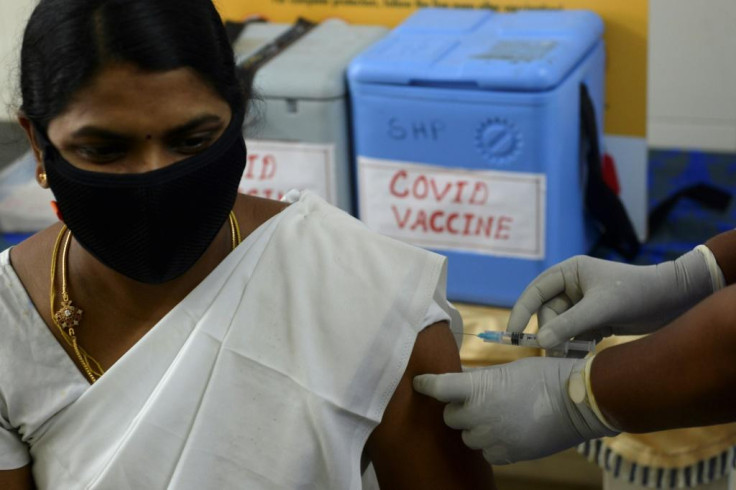
The approval came amid fear in India, as in other countries, about the safety of the shots.
A recent survey of 18,000 people across India found that 69 percent were in no rush to get vaccinated.
"I was excited initially about the vaccine but not anymore because I don't trust it. I am not going to be inoculated," 58-year-old banker Vijaya Das told AFP on Sunday, echoing common apprehension across the country.
"No vaccine is 100 percent effective so what is the guarantee that I won't get the coronavirus after I take the vaccine," added 48-year-old sales manager Suman Saurabh.
Anand Krishnan, a community medicine professor at the All India Institute of Medical Sciences in New Delhi, said authorities needed to publicly release more data about the vaccine trials.
"They have to share the results of the trial and let scientists come to their own conclusion and that way, the vaccine hesitancy would be much less," Krishnan told AFP.
Both vaccines are to be administered in two doses and can be transported and stored at normal refrigeration temperatures.
The Serum Institute of India, the world's biggest manufacturer of vaccines, has said it is making between 50 and 60 million doses a month of the AstraZeneca/Oxford vaccine.
Serum Institute chief executive Adar Poonawalla tweeted following the approvals that the vaccine would be "ready to roll-out in the coming weeks".
Bharat Biotech has yet to complete its Phase 3 trials, but Drugs Controller General Somani said the company's jabs were approved for restricted use in the "public interest as an abundant precaution, in clinical trial mode, to have more options for vaccinations, especially in case of infection by mutant strains".
He told reporters after the briefing that the drug regulator would "never approve anything if there is the slightest safety concern".
Health Minister Harsh Vardhan hit back at opposition lawmakers who expressed fear about the safety of the Bharat Biotech vaccine, tweeting that it was "disgraceful for anyone to politicise such a critical issue".
Copyright AFP. All rights reserved.
This article is copyrighted by International Business Times, the business news leader





















