Uranium isotope ratio throws light on early geological processes of recycling crust material
Researchers have used the uranium isotope ratio to study the recycling of the radioactive substance from the surface of the planet to deep within the mantle.
In the process, they have obtained a better picture of how the Earth's crust was recycled as well as the changing concentrations of oxygen in the atmosphere over the past 600 million years.
Researchers from the University of Bristol, ETH Zurich, Durham, Wyoming and Rhode Island (US), used the 'fingerprint' carried in the ratio of the two uranium isotopes which relates to uranium oxidation processes at the Earth's surface.
"With these measurements, we are able to put a better understanding on the recycling of material back into the Earth's mantle -- the area between the Earth's core and its crust -- back into the deep earth," says Ken Sims, a University of Wyoming professor of geology and geophysics.
"That's because these uranium isotopes are perturbed or changed by the surface processes that are happening. So, we can look at rocks that are coming back out of the mantle and understand this recycling as well as this differentiating of the early Earth."
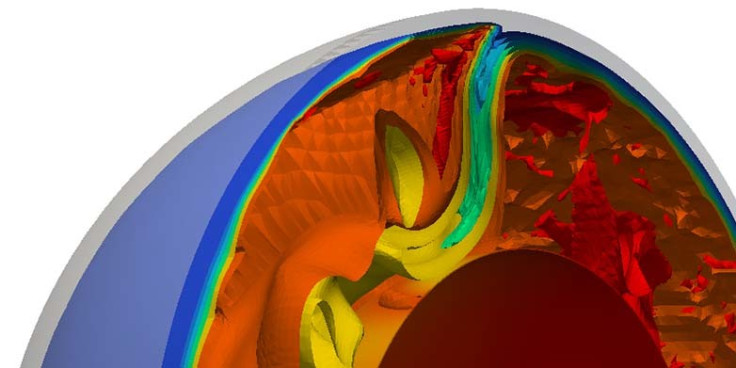
The researchers studied uranium ratio in mid-ocean ridge basalts (MORBs). Mid-ocean ridges are geologically active, with new magma constantly emerging on the ocean floor and spreading away from the ridge.
This was compared with those found in 'hot spots' of ocean island basalts in places such as Hawaii and the Canary Islands where the basalt is made up of material transported from much deeper, less well-mixed part of the mantle.
The isotope ratios are significantly greater for MORBs than for ocean island basalts. The ratios are also higher than that found in meteorites.
The researchers explain that ocean island lava comes from a deeper, less mixed, mantle source which means the uranium added from the surface originates from a much earlier time in Earth's history. These mantle reservoirs formed between 2.4 and 1.8 billion years ago.
In contrast, the uranium isotopic composition of MORB requires the convective stirring of recycled uranium throughout the upper mantle within the past 600 million years.
Study co-author Heye Freymuth of the University of Bristol explains: "Although uranium was incorporated into the oceanic crust since the initial rise in atmospheric oxygen about 2.4 billion years ago, the ocean crust did not incorporate higher amounts of uranium-238 as the oceans did not yet have adequate supplies of oxygen."
Only during the second marked increase in atmospheric oxygen content 600 million years ago did the deep ocean become fully oxidised, allowing the oceanic crust to gain the "fingerprint" of high uranium-238.
So, the uranium isotope ratio of the subducted oceanic crust first differed from the Earth's mantle only after the full oxidation of the oceans.
In an oxygen-free atmosphere, uranium was present in rocks as tetravalent uranium (IV). Once atmospheric oxygen formed, it oxidised uranium to its mobile hexavalent uranium (VI). This more mobile uranium was then released during the weathering and break-down of rocks and transported to the oceans in aqueous form.
When seawater eventually percolated through cracks in the oceanic crust, uranium was absorbed into the oceanic crust.
© Copyright IBTimes 2025. All rights reserved.





















